The Adoption of IEC 60601-1 Around the Globe (Infographic)
October 31, 2017 by CUI Inc -
1 Minute Read
Last updated February 12, 2019
Note: This post was originally published October 31, 2017 and updated on February 12, 2019 to reflect the latest timeline for adoption of IEC 60601-1 worldwide.
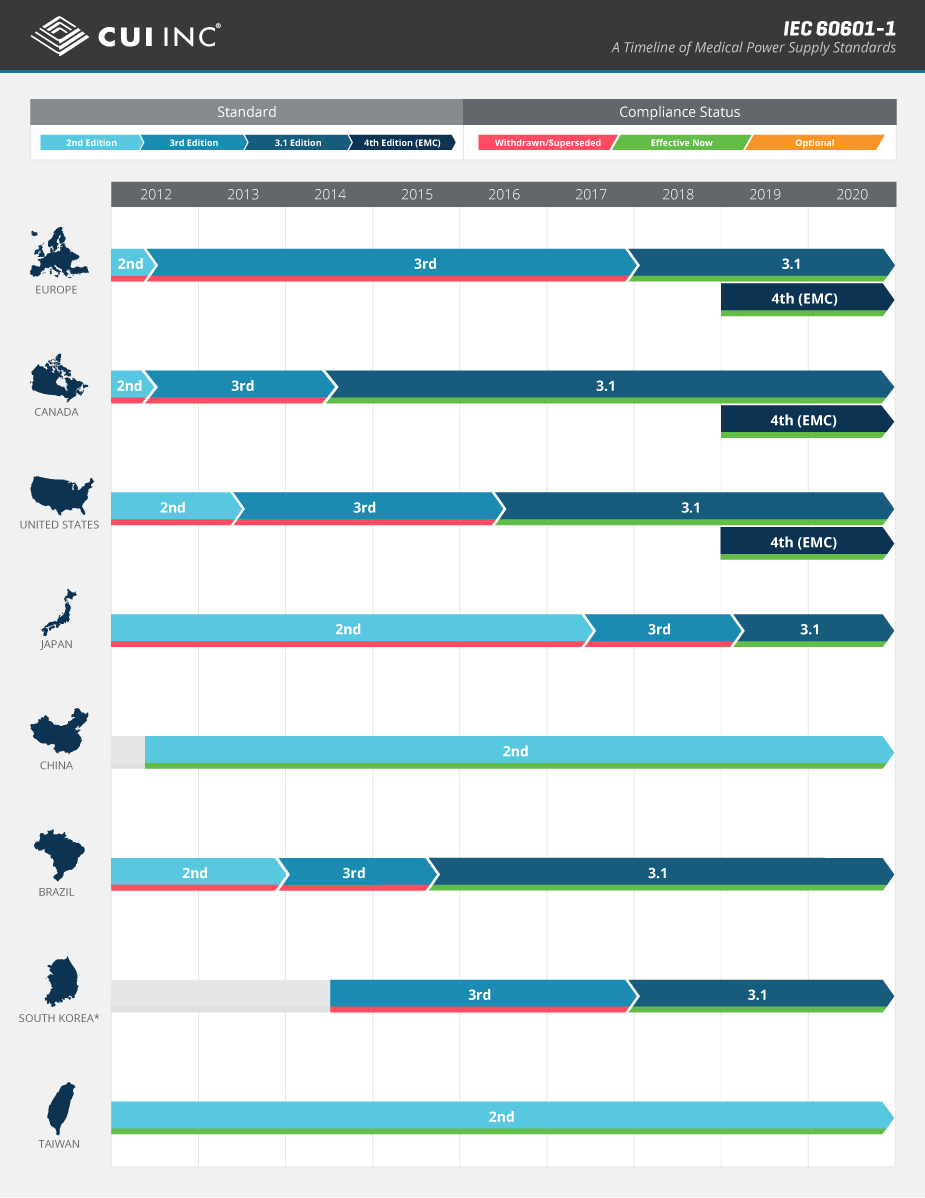
The IEC 60601-1 standard, which provides general requirements that address the basic safety and essential performance of medical electrical equipment, has evolved through a number revisions since its first publication in 1977. Keeping on top of these revisions and new editions of the standard has proven to be a challenge for medical device designers because of their many regional variations and differing adoption timelines around the globe.
With the introduction of the 4th edition of collateral standard IEC 60601-1-2 (“Electromagnetic disturbances – Requirements and tests”) in 2014, the global landscape has become even more unclear. To help illustrate the adoption timeline of IEC 60601-1 as it relates to various territories worldwide, we have developed this country compliance infographic that highlights the evolution of the different editions now and into the future.
You May Also Like
Have comments regarding this post or topics that you would like to see us cover in the future?
Send us an email at powerblog@cui.com